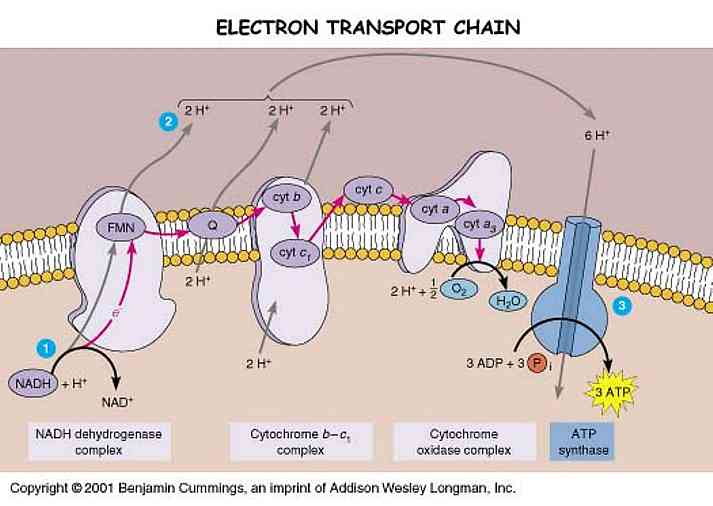
Mitochondria -- affectionately known as the power houses of all cells -- are where energy is produced. There is a series of protein complexes forming the Electron Transport Chain (ETC) which, as their acronym exposes, steal electrons from contributing molecules and convert them into energy and water.
The fully reduced H2O form of oxygen is non-toxic. The various single-electron intermediates between O2 and H2O are ALL toxic free radicals, the so-called Reactive Oxygen Species (ROS). Complex IV (cytochrome oxidase complex) has a gate that transfers electrons directly to O2, reducing it to water without generating ROS. Complexes I and III, however, occasionally allow electrons to escape from the ETC to form ROS.
In Parkinson's disease, Complex I is dysfunctional, and it is thought that much higher concentrations of ROS are produced in the ETC. These attack multiple systems in the mitochondria which eventually lead to the breakdown of cellular DNA, and the cell itself.
Many cases of PD are characterized post mortem by a selective loss of dopamine cells via this mechanism.
The study by Marella et al has used the variant of Complex 1 found in yeast to attempt to quell this rampant ROS formation. Using the rotenone rat model, the group injected the Ndi1 gene via biodegradable microspheres (classy...), and monitored recovery in the substantia nigra pars compacta (SNpc; the primary region of dopamine cell loss) and in behavior.
After 60 days, tissue analysis of Ndi1-injected rotenone rats showed increased staining for viable dopamine cells in the SNpc. Those lesioned rats who did not receive the Ndi1 gene showed significantly fewer stained dopamine cells, and extensive staining with antibody against 8-oxo-dG (indicating oxidative damage to DNA).
My gripe with the study -- in addition to its not being tremendously written -- is that it is lacking in relevant behavioral assessment. The study monitored speed of movement, and the number of rotations in a widely used apomorphine test. The rotations test is normally used in unilaterally lesioned animals (which these were) to indicate preference to rotate in one direction. However, the direction of rotation induced by apomorphine in this study was determined by more factors than the unilateral lesion, which caused the animals to rotate in both directions. Therefore, behavioral data was reported as the "number of animals exhibiting 100% lateralized rotation irrespective of the direction." In my opinion, the behavioral test was severely weakened by this caveat and the group should have employed a quick additional test... like the Whisker test or lateralized grip strength.
This suggests that the Ndi1 gene -- the yeast version of Complex 1 -- was able to compensate for inhibition of Complex 1 by rotenone, decrease ROS activity by serving as an electron transporter, and lessen cell death. If this could be replicated in higher animals, it may prove a viable candidate for clinical trials.
Aside from deficits in writing and behavioral analysis, the story told by this article was fascinating with very intriguing implications. They did their homework, publishing several studies on in vitro activity of the Ndi1 gene and subsequent protein (1, 2) as well as confirming benign effects of introducing a yeast gene in vivo (1, 2) .
Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, & Yagi T (2008). Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson's disease. PloS one, 3 (1) PMID: 18197244